Breast cancer is the most common cancer in women worldwide. It is a complicated illness that needs to be properly diagnosed and treated. In any case, customary strategies for disease recognition, like tissue biopsy, have a few restrictions. A small amount of the tumor’s tissue is taken for a tissue biopsy and examined under a microscope. It can give solid outcomes, however, it is intrusive, exorbitant, tedious, and some of the time not practical when the growth is difficult to reach or too small [1]. Besides, a tissue biopsy may not catch the whole genomic scene of the growth, which can fluctuate starting with one district and then onto the next or change over the long haul because of treatment or illness movement [1].
Liquid biopsy is a promising trial method that includes dissecting an example of body liquid, like blood, pee, or spit, for the presence of malignant growth cells or their hereditary material [2].
Liquid biopsy can offer a few benefits over tissue biopsy, for example:
Early location: Liquid biopsy can distinguish disease cells or DNA before they structure an obvious mass in the body. Early diagnosis and treatment may be possible as a result of this [2].
Less disruptive: A straightforward blood or urine collection is all that is required for liquid biopsy, which does not necessitate surgery or the use of anesthesia. This can diminish the gamble of entanglements and uneasiness for the patient [3].
Simple to repeat: Liquid biopsy can be played out various times over the span of the sickness to screen its encouragement and reaction to therapy. This may be able to provide real-time data on the characteristics and dynamics of the tumor [3].
Comprehensive: By analyzing various components of cancer cells that are released into the circulation, such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), cell-free RNA, exosomes, tumor-educated platelets, proteins, and metabolites, liquid biopsy can capture the heterogeneity and evolution of the tumor [1,4]. The genetic profile, molecular markers, drug resistance, metastatic potential, and prognosis of the tumor can all be gleaned from these components [1,4,5].
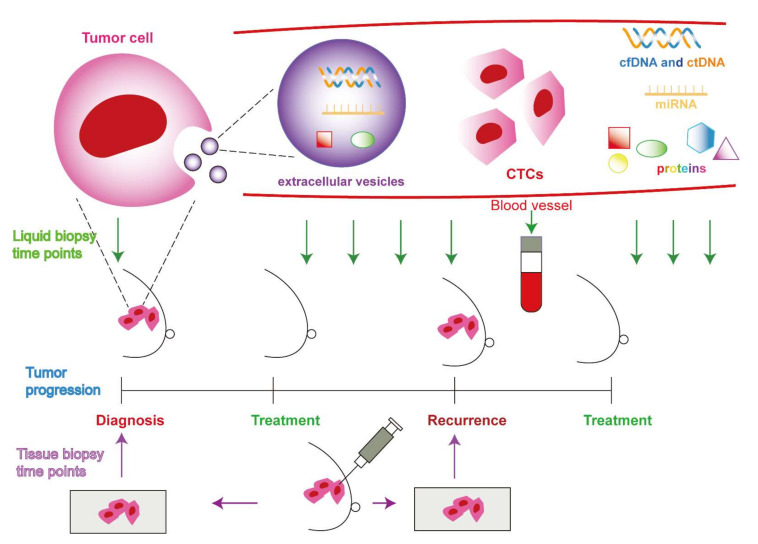
But liquid biopsy also has some problems and limitations that need to be fixed before it can be used in a lot of clinical settings. Here are some examples:
Standardization: Liquid biopsy is as yet an arising method that needs normalized conventions and rules for test assortment, handling, investigation, and translation. [1,4] Different platforms and methods may produce different outcomes and accuracy.
Sensitivity: Fluid biopsy will most likely be unable to identify lacking degrees of disease cells or DNA in the body liquid. This might prompt misleading adverse outcomes or missed analysis [1,4].
Specificity: It’s possible that a liquid biopsy won’t be able to tell the difference between DNA or cancer cells from different places or sources. This might prompt bogus positive outcomes or misdiagnosis [1,4].
Moral issues: Concerns regarding informed consent, data security, privacy, and the disclosure of results may arise with liquid biopsy. Patients might be taught about the advantages and dangers of fluid biopsy and their suggestions for their well-being and prosperity [2].
In conclusion
Liquid biopsy is a promising method that can be used in conjunction with tissue biopsy to detect breast cancer. Early detection, ease of repeatability, comprehensive tumor information, and less invasiveness are some of its potential benefits. However, before it can be widely used in clinical practice, it must also overcome some obstacles and limitations. To improve liquid biopsy’s standardization, sensitivity, specificity, and ethical aspects, additional research and development are required.
Our Knowledge
Celer Diagnostics scientists have developed a liquid biopsy kit called Celer Kit that is designed to detect and even predict breast cancer. Celer Kit is based on extracellular vesicles (EVs), which are small membrane-bound particles that contain constituents (protein, DNA, and RNA) of the cells that secrete them. EVs can reflect the state and activity of the original cells and can be used as biomarkers for cancer diagnosis.
Celer Kit is fast, cost-effective, and minimally invasive. It requires only 5 mL of blood from the patient and can obtain results in less than 2 hours. Celer Kit observes 7 biometrics, including ASPH protein, in blood samples, whereas 5 biometrics are observed in the physical biopsy. ASPH protein is a novel biomarker that is highly expressed in breast cancer cells and plays a role in tumor growth and metastasis.
References
- Liquid biopsy in breast cancer: A comprehensive review – Alimirzaie – 2019 – Clinical Genetics – Wiley Online Library
- link.springer.com
- What Is a Liquid Biopsy? (webmd.com)
- Current and Developing Liquid Biopsy Techniques for Breast Cancer – PubMed (nih.gov)
- Biopsy: Types of biopsy procedures used to diagnose cancer