“Breast cancer” is the most common cancer and the leading cause of cancer death among women worldwide. Early diagnosis and treatment are crucial for improving the survival and quality of life of breast cancer patients. However, current diagnostic methods, such as mammography and tissue biopsy, have some limitations, such as invasiveness, low sensitivity, and inability to capture the heterogeneity and dynamics of the tumor.
Liquid biopsy is a novel technique that allows the detection and monitoring of cancer using a blood or fluid sample, instead of a solid tissue sample. Liquid biopsy can reveal various biomarkers that are shed by tumors into the circulation, such as circulating tumor cells, circulating tumor DNA, and extracellular vesicles (EVs).
EVs are tiny membrane-enclosed particles that are released by all kinds of cells, including cancer cells. EVs carry lipids, proteins, and nucleic acids that reflect the characteristics and status of their parent cells. EVs can protect their cargoes from degradation in the extracellular environment, making them stable and reliable biomarkers for liquid biopsy.
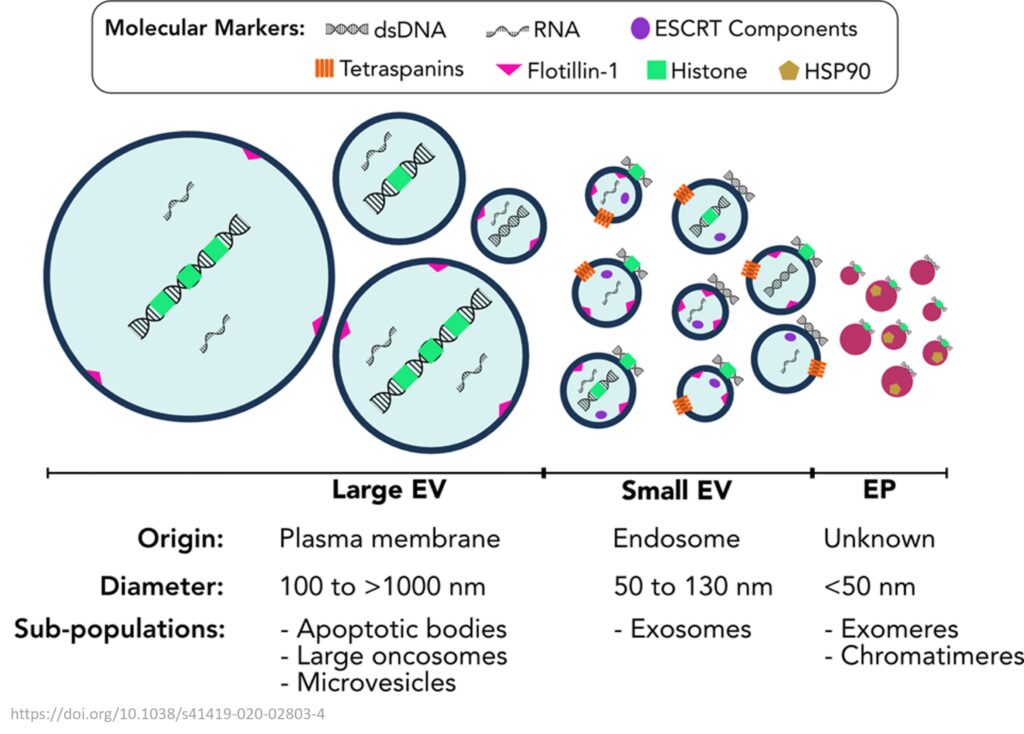
EVs have several advantages over other liquid biopsy analytes, such as:
- They are abundant and easily accessible in various body fluids, such as blood, urine, saliva, and cerebrospinal fluid.
- They can capture the heterogeneity and diversity of the tumor, as well as its interaction with the microenvironment and the immune system.
- They can provide information on both protein and nucleic acid levels, as well as their combinations, enabling a comprehensive and multiplexed analysis of the tumor.
EVs have been shown to be useful for breast cancer diagnosis, prognosis, treatment selection, and response evaluation. For example, EVs can reveal the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67, which are important biomarkers for breast cancer subtyping and therapy decisions [1]. EVs can also detect mutations and alterations in genes such as PIK3CA, TP53, and BRCA1/2, which are associated with breast cancer risk, progression, and resistance [2]. Moreover, EVs can monitor the response to chemotherapy, endocrine therapy, targeted therapy, and immunotherapy by measuring changes in their quantity, composition, and function [3].
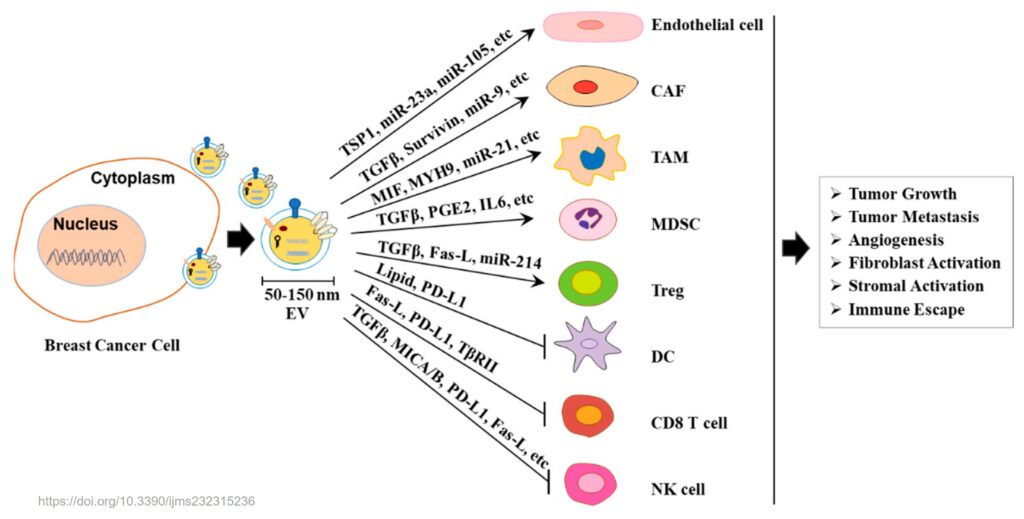
Several methods have been developed to isolate and analyze EVs from liquid biopsies. These methods include ultracentrifugation, size-exclusion chromatography, immunoaffinity capture, microfluidics, nanotechnology, and spectroscopy [4]. However, these methods also face some challenges, such as low specificity, high cost, low throughput, and lack of standardization. Therefore, more research is needed to optimize and validate these methods for clinical applications.
In conclusion, EVs are promising biomarkers for liquid biopsy in breast cancer. They can provide valuable information about tumor biology and dynamics that can help improve diagnosis and treatment. However, more studies are needed to overcome the technical and clinical challenges of EV-based liquid biopsy.
References:
- Liu J., Chen Y., Pei F., Zeng C., Yao Y., Liao W., Zhao Z. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. BioMed Research International. 2021;2021:6611244. doi: 10.1155/2021/6611244.
- Wu H.-J., Chu P.-Y. Current and Developing Liquid Biopsy Techniques for Breast Cancer. Cancers. 2022;14(9):2052. doi: 10.3390/cancers14092052.
- Kim S.-H., Lee J.-H., Kim S.-I., Lee J.-E., Nam S.-J., Park Y.-H., et al. Circulating extracellular vesicles are effective biomarkers for diagnosis of early-stage breast cancer using next-generation sequencing. EBioMedicine. 2021;68:103397. doi: 10.1016/j.ebiom.2021.103397.
- Lin A.A., Nimgaonkar V., Issadore D., Carpenter E.L. Extracellular Vesicle-Based Multianalyte Liquid Biopsy as a Diagnostic for Cancer. Annual Review of Biomedical Data Science. 2022;5(1):269–292. doi: 10.1146/annurev-biodatasci-122120-113218.